Research Overview
We aim to develop novel functions and properties for inorganic and complex materials by utilizing instrumental analysis. Specifically, by analyzing the molecular and crystal structures and electronic states of metal oxide clusters, porous metal complexes, and metal nanoparticles, we seek to understand the structure-function correlation and apply it to the adsorption, conduction, and conversion of molecules and ions.
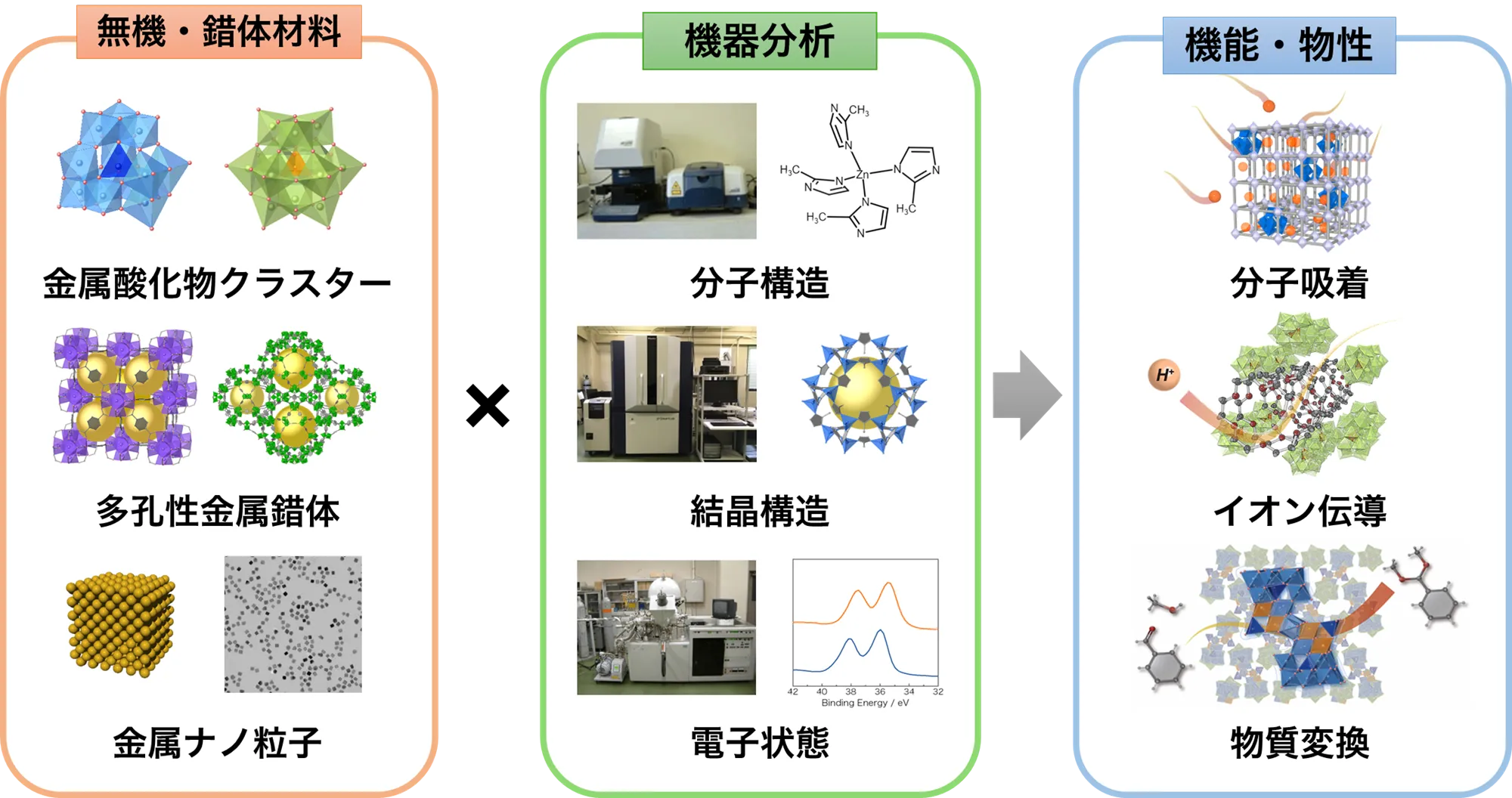
Main Research Topics
Adsorption Function Using Nanospace
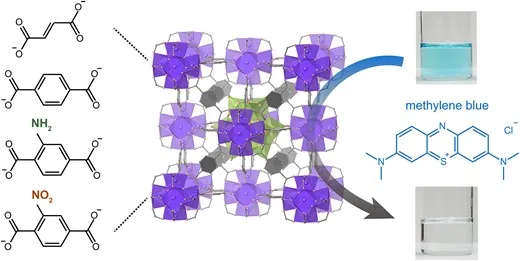
Porous Coordination Polymers (PCP) and Metal-Organic Frameworks (MOF) are porous materials composed of metal ions and bridging ligands, containing “nano spaces” within their structures. We utilize the nano spaces in PCP/MOF as adsorption sites for carbon dioxide (CO2), known as a greenhouse gas, and hydrogen (H2), a promising next-generation energy carrier ( Chem. Sci. 2016 , Chem. Lett. 2019 ).
To expand the versatility of PCP/MOF as adsorption sites, we have reported the adsorption functions for positively charged Cs+ ions and methylene blue cations by introducing negatively charged metal oxide clusters into the PCP/MOF structure ( Small 2024 , Bull. Chem. Soc. Jpn. 2024 ).
Development of Ion Conductors and Understanding of Molecular Motion
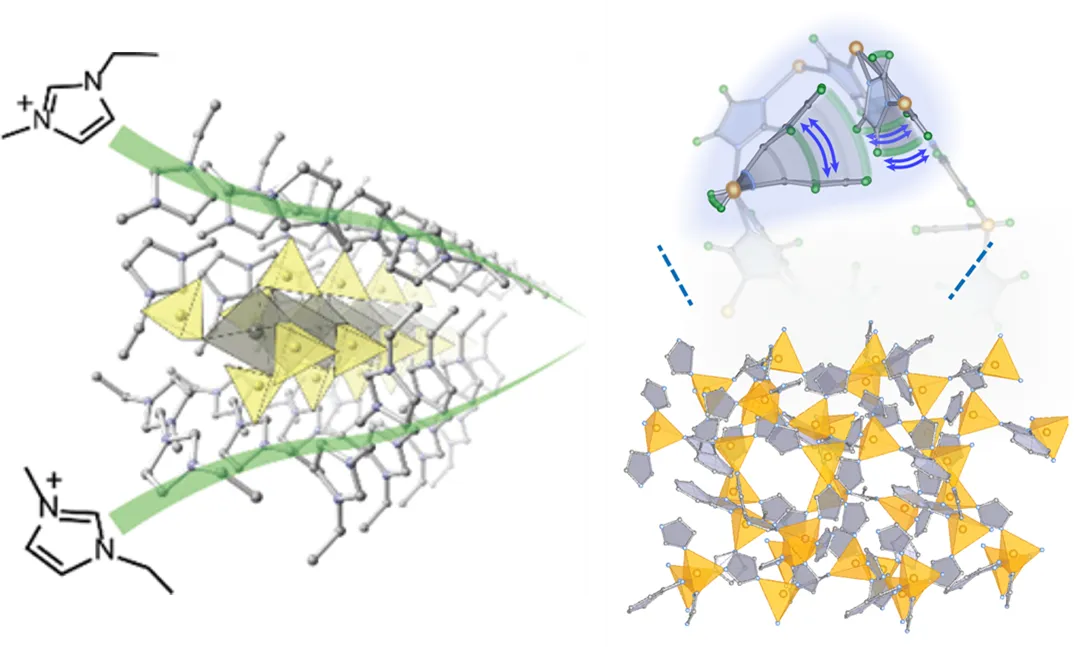
Ion conduction phenomena in solids are important phenomena that are key to a wide range of functional expressions such as battery electrolytes and sensors. We are attempting to develop new ion conductors using metal complexes and metal oxide clusters ( Chem. Mater. 2016 , Nanoscale 2021 , Coord. Chem. Rev. 2022 ). We are also advancing research on understanding molecular motion, which is known to be strongly correlated with ion conduction ( Chem. Commun. 2019 , J. Am. Chem. Soc. 2024 ).
Material Conversion Using Nanomaterials
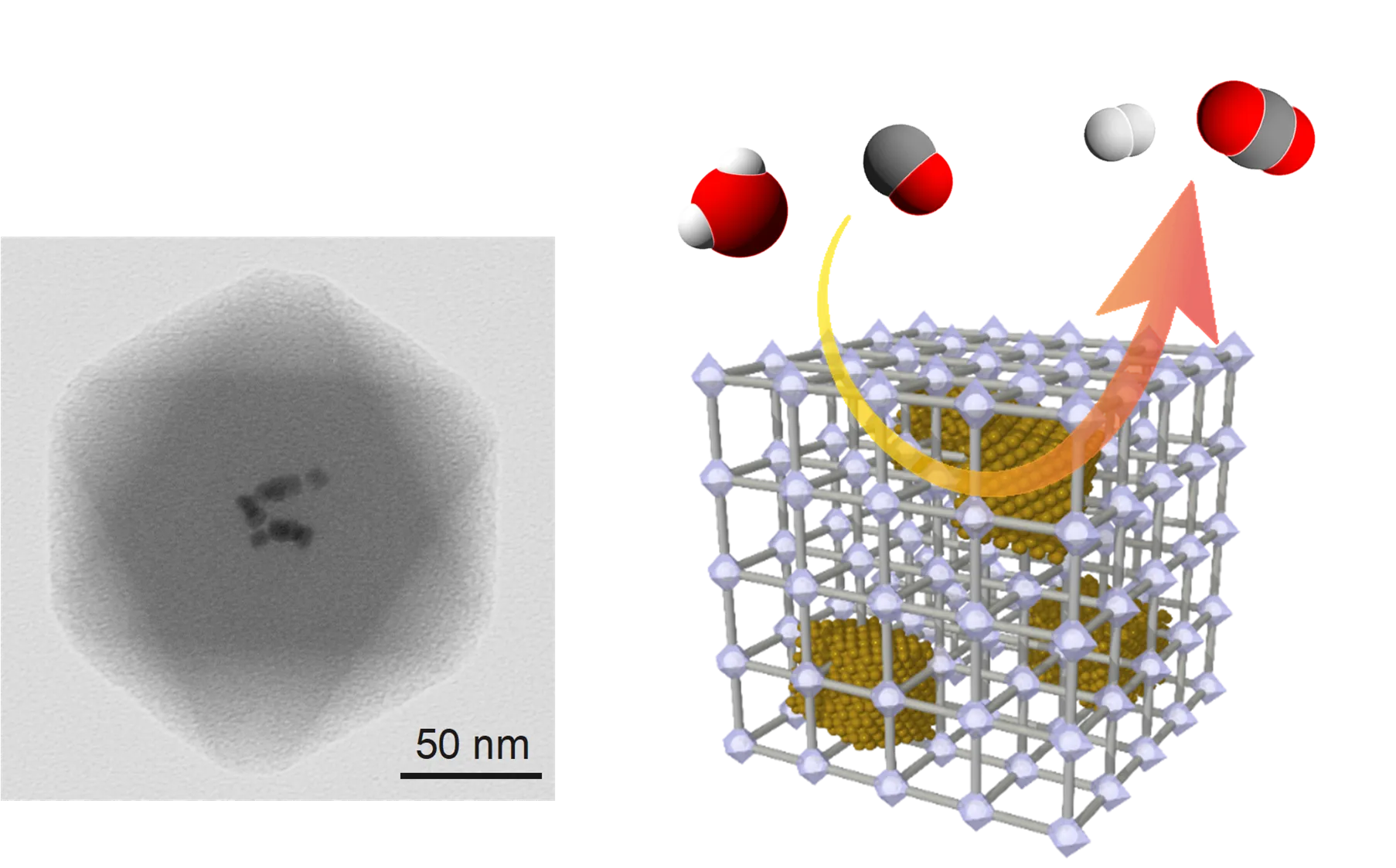
In the chemical industry, the development of catalytic materials to convert raw materials into high-value-added compounds is valuable. We have reported hydrogen generation catalysts using nanocomposites with metal nanoparticles embedded in PCP/MOF ( Angew. Chem. 2019 , Nano Lett. 2020 ) and acid catalysts using metal oxide clusters ( Chem. Commun. 2021 , Chem. Commun. 2022 , Chem. Catal. 2023 , Chem. Sci. 2024 ).